Guidelines for Supply Triage During Visudyne (Verteporfin) Shortage

Created on July 16, 2021
Revised September 19, 2025
Visudyne is an essential therapeutic agent for the treatment of specific sight-threatening
retinal and choroidal diseases, including choroidal tumours, anti-VEGF resistant neovascular
age-related macular degeneration (nAMD) and central serous choroidopathy (CSC). There is
an active global shortage of Visudyne, and the next resupply is not expected to be available in
Canada until the end of June 2026 (see: www.drugshortagescanada.ca).
The Canadian Retina Society (CRS) and Canadian Ophthalmological Society (COS) are working
with Health Canada’s Drug Shortages Unit on this issue. Further, the CRS and the COS are
suggesting the following mitigation strategies and suggest that the remaining supply of
Visudyne be prioritized as follows:
- Priority 1: For the full course of treatment and management of sight threatening Ocular Tumours
- Priority 2: For the treatment of non-resolving CSC
- Priority 3: For the treatment of anti-VEGF resistant nAMD
The CRS and COS provide this information for guidance and urge all treating Ophthalmologists
to collaborate with their local and regional oncology and retinal specialist groups to ensure
careful triage of this agent to those patients in most dire need.
Joint Statement: Betoptic S Discontinuation

Betoptic S (betaxolol 0.25%) is being discontinued globally by Novartis. Existing Canadian supplies are expected to run out by the end of this year. There is no other marketed betaxolol eye drop in Canada. Novartis has indicated that this is a global decision and will not be reversed. The discontinuation has already occurred elsewhere across the globe. We have created a small document for our colleagues in anticipation of this change.
Betaxolol and Timolol provide similar efficacy in treating glaucoma.1 However, timolol (a non-selective beta blocker) can adversely affect pulmonary function whereas betaxolol (a selective B-1 blocker) has less impact on pulmonary function.2 Therefore, betaxolol has been recommended as the topical beta blocker of choice in patients with pulmonary disease (e.g. asthma, COPD). While betaxolol is not widely prescribed, it remains a part of glaucoma control for some patients.
We hope providers will guide patients through this drug discontinuation. Alternative management strategies may include switching to another class of topical glaucoma medication (e.g. prostaglandin analogue, carbonic anhydrase inhibitor, alpha agonist) if no contraindication exists,3 increasing ocular availability while decreasing systemic absorption of other therapies by eyelid closure or nasolacrimal occlusion,4 laser trabeculoplasty,5 minimally invasive glaucoma surgery (MIGS) or minimally invasive bleb surgery (MIBS),6 or incisional glaucoma surgery.7 Patient needs and availability of treatment will be unique across the country. We thank you, the providers, for continuing to manage excellence in care for all Canadians.
- Stewart RH, Kimbrough RL, Ward RL. Betaxolol vs timolol. A six-month double-blind comparison. Arch Ophthalmol. 1986 Jan;104(1):46-8
- Schoene RB, Abuan T, Ward RL, Beasley CH. Effects of topical betaxolol, timolol, and placebo on pulmonary function in asthmatic bronchitis. Am J Ophthalmol. 1984 Jan;97(1):86-92
- Li T, Lindsley K, Rouse B, Hong H, Shi Q, Friedman DS, Wormald R, Dickersin K. Comparative Effectiveness of First-Line Medications for Primary Open-Angle Glaucoma: A Systematic Review and Network Meta-analysis. Ophthalmology. 2016 Jan;123(1):129-40
- Flach AJ. The importance of eyelid closure and nasolacrimal occlusion following the ocular instillation of topical glaucoma medications, and the need for the universal inclusion of one of these techniques in all patient treatments and clinical studies. Trans Am Ophthalmol Soc. 2008;106:138-45
- Li X, Wang W, Zhang X. Meta-analysis of selective laser trabeculoplasty versus topical medication in the treatment of open-angle glaucoma. BMC Ophthalmol. 2015 Aug 19;15:107
- Nichani P, Popovic MM, Schlenker MB, Park J, Ahmed IIK. Microinvasive glaucoma surgery: A review of 3476 eyes. Surv Ophthalmol. 2021 Sep-Oct;66(5):714-742
- Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL; Tube versus Trabeculectomy Study Group. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012 May;153(5):789-803.e2
Joint Position Statement on Plasma Technology for Blepharoplasty and the Role of Social Media in Cosmetic Procedures
The Canadian Ophthalmological Society (COS) and the Canadian Society of Oculoplastic Surgery (CSOPS) are dedicated to ensuring the highest standards of patient care and professional practice within the fields of ophthalmology and oculoplastic surgery. The growing use of plasma technology in blepharoplasty and its promotion through various media, including social media influencers, calls for clear guidance and information for both our members and the public. The joint position statement is available to read below:
To access the French version, click here | Pour accéder à la version française, cliquez ici
2025 Federal Election Members Advocacy Toolkit
For the French version, follow this link | Pour la version française, suivez ce lien – https://www.cosprc.ca/fr/trousse-de-sensibilisation-des-membres-elections-federales-de-2025/
Joint Position Statement on the Use of Patented Microcurrent Technology for Dry AMD
The Canadian Ophthalmological Society (COS) and Canadian Retina Society (CRS) advise caution when considering a new microcurrent treatment for dry age-related macular degeneration (AMD). While early studies suggest it may help, there isn’t enough long-term evidence to confirm its safety or effectiveness. Retinal specialists, given their extensive expertise in managing age-related macular degeneration, recommend that patients and doctors discuss the treatment carefully and understand its limitations before deciding. The joint position statement is available to read below:
Vision Health Conference 2024
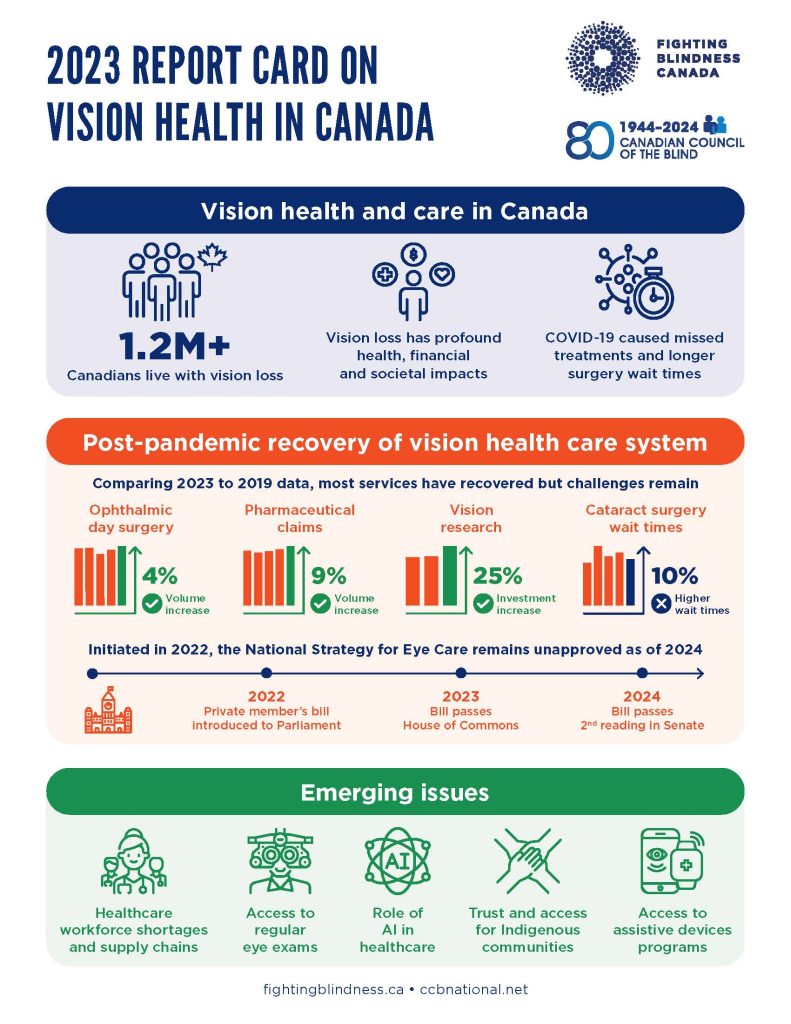
Report Card: The Post-Pandemic State of Vision Health in Canada
Date: Wednesday, October 30, 2024
Time: 10 am – 2 pm
Location: Toronto Reference Library
789 Yonge Street, Toronto, Ontario, M4W 2G8
Bram & Bluma Appel Salon (2nd floor)
This conference will present results from the newly-released Report Card Part 2, undertaken by the Canadian Council of the Blind (CCB) and Fighting Blindness Canada (FBC), which is a follow-up to the Report Card Part 1, released in October 2022.
The report provides an update on the current state of vision health in Canada and what we can anticipate as we move forward from the post-pandemic era. The report offers insights into the key issues affecting vision health and the community of people living with vision loss.
This is a hybrid event, meaning the same content will be offered at the same time.
For in-person registration, click HERE.
For online registration, click HERE.
You can view the 2023 Report Card below!
CJO: August 2024 Issue Highlights
The August 2024 CJO is now available online. Here are some of the highlights:
Resident Perspectives + visual abstract: Our talented team of residents have summarized 3 articles from this issue with a focus on what’s most relevant to ophthalmology learners here in Canada and around the globe. In addition, our August visual abstract provides a visual summary of an article on the Epidemiology of ocular emergencies in a large Canadian eye centre.
Reviews and Original Research Articles:
- Advances in conjunctival melanoma: clinical features, diagnostic modalities, staging, genetic markers, and management
- Advances in multimodal imaging for diagnosis of pigmented ocular fundus lesions
- Research productivity of first-year Canadian ophthalmology residents: a 12-year trend
- Visualization of preretinal membranes using trypan blue in patients with traction retinal detachments
- Artificial intelligence chatbot performance in triage of ophthalmic conditions
- Retinal and choroidal microvascular changes during pregnancy detected with OCTA
Research Letters, Photo Essays and Case Reports:
- Performance of three artificial intelligence chatbots on Ophthalmic Knowledge Assessment Program materials
- Scleral patch graft for emergency open-sky repair
- Cavitary ciliary body melanoma with extensive pigment dispersion
- Acute macular neuroretinopathy following uncomplicated epiretinal membrane removal
- Bilateral circumscribed choroidal hemangiomas in 2 patients: a rare finding
- 532-nm laser for sub-internal limiting membrane hemorrhage associated with retinal macroaneurysms
- Syndromic PRD: case report of McArdle retinopathy and review of literature
Follow the CJO on social media:
Facebook: CanJOphth
Instagram: @cjo_jco
LinkedIn: CJO – JCO
Twitter: @CanJOphth
Understanding Ophthalmologists’ Perspectives on Biosimilars
Background
A needs assessment was conducted to better understand the needs of Canadian ophthalmologists in the context of biosimilars. Biosimilars are biotherapeutic agents that aim to replicate the efficacy and safety profile of their reference biologic counterparts. They undergo rigorous testing to ensure comparable safety, efficacy, and quality. Biosimilars hold the potential to enhance patient access to essential medications and foster competition within the healthcare market.
The purpose of the needs assessment was to determine the necessity of educational programs and other assets to support ophthalmologists in effectively navigating biosimilars.
Ophthalmologists’ Knowledge and Practices
Ophthalmologists are becoming more aware of biosimilars but there is still some hesitancy in adopting their widespread usage. In order to better understand their perspectives, COS conducted 2 surveys – one in English and one in French.
Online surveys were distributed to ophthalmologists across Canada. There was an overall completion rate of 76% (95 complete, 30 partial responses out of 125 total). The English survey had representation from multiple provinces, with the highest being Ontario. The French survey respondents were 100% from Quebec. Despite some provincial skewing, participation was geographically diverse offering a comprehensive view.
The findings revealed that while awareness of biosimilars for anti-VEGF treatments stood at 61.1%, actual usage was notably lower, with only 26.1% of respondents having utilized biosimilars. Preparedness to incorporate biosimilars into practice varied among ophthalmologists, with larger studies, longer-term analyses, and real-world evidence identified as factors that could increase comfort levels with biosimilar adoption.
Interest in educational initiatives was pronounced, with respondents expressing a desire for webinars, online seminars, clinical practice guidelines, and increased educational meetings or journal clubs. However, concerns persisted regarding limited understanding of safety and efficacy, restricted access to information, and uncertainty surrounding regulatory standards and approval processes.
Perceptions and Concerns about Biosimilars
Ophthalmologists voiced nuanced opinions regarding the approval process and the likelihood of biosimilar adoption. Notably, 80% of respondents advocated for implementation of larger clinical trials to ensure the safety and efficacy of biosimilar drugs. However, views on the likelihood of adopting biosimilars in the near future were mixed.
Open-ended responses underscored the diversity of opinions among ophthalmologists, with concerns ranging from safety and efficacy to the potential for cost savings and the necessity for additional evidence-based data. The influence of medication cost and the presence of patient support programs moderately affected decision-making processes. Uncertainty surrounding regulatory standards and approval processes emerged as a common concern.
Conclusions and Next Steps
The needs assessment’s key findings emphasize the imperative for further education, larger clinical trials, and real-world evidence to assuage concerns and enhance ophthalmologists’ comfort levels with biosimilars. These perspectives have tangible implications for decision making processes and the potential adoption of biosimilar drugs in ophthalmology.
Recommendations include the development of comprehensive educational initiatives, collaboration for larger studies, and the generation of real-world evidence to address ophthalmologists’ concerns effectively. Additionally, the establishment of an educational toolkit tailored to biosimilar usage in ophthalmic practice is recommended for guiding informed decision-making and ensuring patient safety.
This needs assessment was completed with an unrestricted educational grant with COS and Apotex, Biocon & Biogen and was planned to achieve scientific integrity, objectivity and balance.
Summary of Data
Awareness and Usage of Biosimilars
- 61.1% of ophthalmologists surveyed are aware of biosimilars for retinal conditions
- However, only 26.1% are currently using them in their practice
Likelihood of Using Biosimilars
- 51% said they are likely to start using biosimilars in their practice in the near future
- 49% said they are not likely to start using them
Factors to Increase Comfort Level with Biosimilars
- More educational meetings/journal clubs (56%)
- Real-world studies (46.2%)
- Longer-term studies (38.5%)
- Larger studies (29.7%)
Educational Interests
Ophthalmologists expressed interest in various educational modalities to increase their knowledge of biosimilars
- Webinars/online seminars (57.1%)
- Clinical practice guidelines (50.5%)
- Peer-reviewed journal and articles (39.6%)
- Case-based discussions/grand rounds (37.4%)
- Mentorship programs (26.4%)
To view the sources for this needs assessment, you can download them here:
We would like to take the opportunity to thank the scientific planning committee for conducting this needs assessment. To view their profiles, you can download the document here:
Corneal Neuropathic Pain General Information for Patients and Providers

March, 2023
Definition
Corneal neuropathic pain, or corneal neuralgia, may be defined as chronic eye pain lasting more than three months that is not responsive to standard measures, and severe enough to affect daily living. The phenomenon is sometimes described after ocular surgery, including corneal and intraocular, but can occur after any type of eye trauma.
The condition is not well understood and there is no agreement on the diagnostic criteria, causes, incidence, nor treatment.
Pathophysiology
The V1 ophthalmic division of the trigeminal nerve contains nociceptors that respond to a variety to stimuli, including mechanical force and temperature. When stimulated, a signal transduction is transmitted and processed in the pain regions of the cerebral cortex. It is hypothesized that repeated damage to the cornea and its corresponding neuronal pathway results in a hyper‐inflammatory state, which leads to maladaptive sensitization either peripherally at the cornea or centrally at postsynaptic junctions.
Therefore, it may be useful to assess and manage corneal neuropathic pain as either peripheral or central pain sensitization.
Symptoms
Symptoms include, but are not limited to:
- Chronic, unrelenting eye pain
- Dry eye syndrome without signs, which is non‐responsive to standard treatment
- Light sensitivity, burning, foreign body sensation
- Symptom severity has resulted in suicide
Signs
- “Pain without stain”,
- Punctate keratopathy
- No signs may be present
Associated Conditions
- Central somatic sensitivity syndrome
- Fibromyalgia
- Autoimmune disease
- Chronic migraine and headache
- Persistent Post‐operative Pain Disorders (PPP)
Investigations
- Patients’ response to topical anesthetic may be useful in determining if the corneal neuropathic pain is a central or peripheral cause. A response of decreased pain with local anesthetic is suggestive of peripheral etiology.
In vivo Confocal microscopy
The sub‐basal nerve density has been reported to be significantly reduced in corneal neuropathic pain compared with control subjects. Other features described are activated keratocytes and spindle, lateral and stump microneuromas. However, the significance of these findings is unclear, as similar changes can be observed following uncomplicated PRK and LASIK. Therefore, the current role of confocal microscopy in these patients is unclear, and it is available on a limited basis in Canada mostly at academic centers.
Treatment Goals
- Regenerate corneal nerves and reduce inflammation
- Patients with predominantly peripheral sensitization likely have a better outcome than those with central sensitization
- Maximize ocular surface management as with severe dry eye
- Frequent preservative‐free artificial tears, limited use of topical steroids, night‐ time gels, topical cyclosporine
- Most patients will have had this treatment prior to referral with failure to respond
Additional Treatment Options
- Autologous serum drops
- Amniotic membrane
- Neurostimulation
- Corneal growth factors
- Neuromodulation
- Scleral contact lenses
- Systemic treatment may be necessary such as gabapentin, pregabalin, amitriptyline, and low‐dose naltrexone
- Referral to neuro‐ophthalmologist/neurologist/pain specialist. Resources are available in Canada for additional consultation and management
Outcomes
There is a high degree of variability in patient outcomes, with some patients suffering long‐term disability from the symptoms while others show complete resolution after 1 to 2 years. Information in this regard is anecdotal but it is advisable to take all possible measures to manage the disease, as long standing corneal neuralgia tends to be associated with worse patients outcomes.
References
Aggarwal S, Kheirkhah A, Cavalcanti BM, et al. Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. The Ocular Surface. 2015;13(3):250‐262. doi:10.1016/j.jtos.2015.01.005
Crane AM, Levitt RC, Felix ER, Sarantopoulos KD, McClellan AL, Galor A. Patients with more severe symptoms of neuropathic ocular pain report more frequent and severe chronic overlapping pain conditions and psychiatric disease. British Journal of Ophthalmology. 2017;101(2):227‐231. doi:10.1136/bjophthalmol‐2015‐308214
Galor A, Covington D, Levitt AE, et al. Neuropathic Ocular Pain due to Dry Eye Is Associated With Multiple Comorbid Chronic Pain Syndromes. The Journal of Pain. 2016;17(3):310‐318. doi:10.1016/j.jpain.2015.10.019
Kundu G, Shetty R, D’Souza S, et al. A novel combination of corneal confocal microscopy, clinical features and artificial intelligence for evaluation of ocular surface pain. PLoS One. 2022;17(11):e0277086. doi:10.1371/journal.pone.0277086
Levitt, A.E., Galor, A., Weiss, J.S. et al. Chronic dry eye symptoms after LASIK: parallels and lessons to be learned from other persistent post‐operative pain disorders. Mol Pain 11, 21 (2015). https://doi.org/10.1186/s12990‐015‐0020‐7
Moshirfar M, Benstead EE, Sorrentino PM, Tripathy K. Ocular Neuropathic Pain. In: StatPearls. StatPearls Publishing; 2022. Accessed January 21, 2023. http://www.ncbi.nlm.nih.gov/books/NBK542282/
Neuropathic Corneal Pain ‐ ClinicalKey. 2023.https://www.clinicalkey.com/#!/content/playContent/1‐s2.0‐S0161642017306127
Ocular Neuropathic Pain ‐ EyeWiki. https://eyewiki.aao.org/Ocular_Neuropathic_Pain
Pakravan M, Roshani M, Yazdani S, Faramazi A, Yaseri M. Pregabalin and Gabapentin for Post‐Photorefractive Keratectomy Pain: A Randomized Controlled Trial. European Journal of Ophthalmology. 2012;22(7_suppl):106‐113. doi:10.5301/ejo.5000143
Rosenthal P, Borsook D. Ocular neuropathic pain. Br J Ophthalmol. 2016;100(1):128‐134. doi:10.1136/bjophthalmol‐2014‐306280



